According to relevant data, the total incidence of birth defects in China is about 5.6%, and there are about 900,000 new birth defects every year [1]! Birth defects not only affect the health and life quality of children, but also affect the stability and happiness of the entire family, and affect the quality of the birth population and the health stock of human resources in the entire country.
What are birth defects?
Birth defects are abnormalities in body structure, function, or metabolism that occur before a baby is born, caused by genetic or environmental factors. Current studies have found that chromosomal abnormalities account for a huge proportion of defective children, especially fetal chromosomal aneuploidy diseases. Among them, there are four main types of trisomy 13 syndrome, trisomy 18 syndrome, trisomy 21 syndrome and abnormal number of sex chromosomes. According to statistics, these 4 kinds of chromosomal abnormalities account for 80% to 95% of prenatal fetal chromosomal abnormalities [2], see Figure 1.
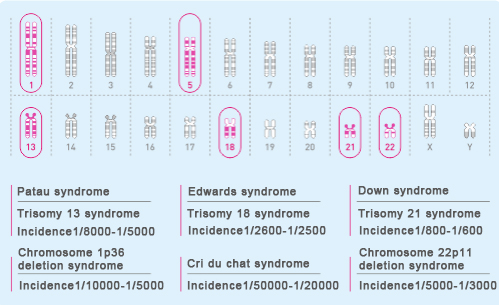
Figure 1 Incidence of common abnormal diseases of fetal chromosomal aneuploidy
Prenatal screening and diagnosis is the only way to avoid birth defects
In the actual clinical application process, a series of screening work will be carried out in combination with different pregnancy cycles of pregnant women. Maternal serum screening (Down syndrome screening) is usually performed in the first and second trimesters, combined with ultrasound imaging, to measure the nuchal translucency (NT) at the corresponding stage, and to screen for fetal malformations.
Figure 2 Medical service items related to prenatal examination
Since the above-mentioned routine screening has a certain probability of detection, invasive prenatal diagnosis was usually required in the past to confirm the diagnosis when there were indications of risk. The gold standard for clinical diagnosis of fetal chromosomal abnormalities is invasive prenatal diagnosis, including chorionic villus biopsy, amniocentesis, and umbilical cord blood puncture, but invasive prenatal diagnosis has the risk of fetal loss and infection [3]. Pregnant women may therefore be unable or refuse invasive prenatal diagnosis, leading to miscarriage or fetal birth defects.
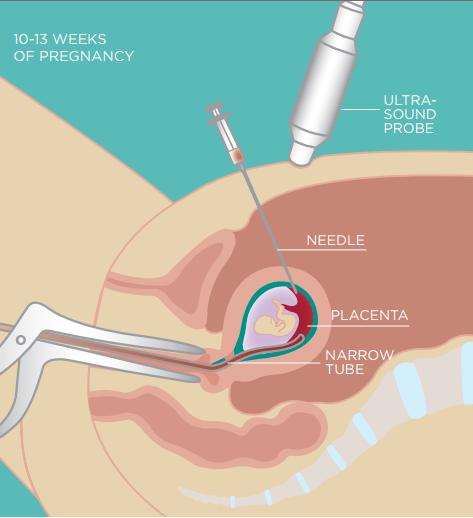
Figure 3 Chorionic villus biopsy
The development and application of NIPT technology reduces the probability of needing invasive prenatal diagnosis and benefits most pregnant women
NIPT technology is a prenatal detection method that analyzes cell-free fetal DNA fragments in maternal plasma by next-generation sequencing (NGS) technology to determine whether the fetus has genetic variation [4]. Cell free fetal DNA (cff DNA) appears in maternal peripheral blood at 4 weeks of gestation, and its concentration increases with gestational weeks, and can account for 10% of the total cell-free DNA in plasma at 11-13 weeks [5]. NITP is to detect fetal DNA by collecting peripheral blood of pregnant women and extracting cell-free DNA from it.
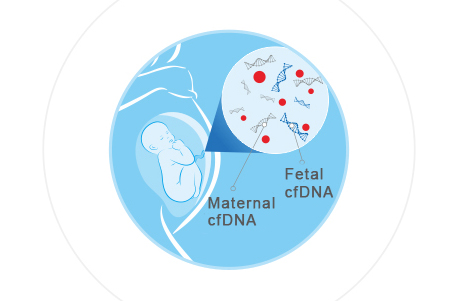
Figure 4 Inspection by cell-free fetal DNA
As a screening technique, NIPT is currently widely used in clinical practice. Compared with the low detection rate and high false positive rate of traditional screening techniques, it has the advantages of high accuracy, high sensitivity and high specificity. Mata analysis shows that the detection rates of T13, T18, and T21 of NIPT are 99.0%, 98.2% and 99.7%, respectively [5].
The current clinical application of NIPT is mainly when routine early screening or familial genetic factors indicate high-risk indications, it is recommended that pregnant women undergo NIPT first, and comprehensively determine whether pregnant women are advised to undergo invasive prenatal diagnosis based on the test results.
This comprehensive combination of screening and diagnosis has significantly reduced births with birth defects, unnecessary invasive prenatal diagnosis, and miscarriages due to invasive prenatal diagnosis.
As technology developed, NIPT also began to be used to screen for other aneuploidies and chromosomal microdeletions and microduplications.
Routine NIPT experimental process
Common NIPT technology experimental process includes: NIPT sample collection, NIPT sample reception and storage, NIPT sample DNA extraction and purification, library construction and purification, Pooling library preparation and computerization, sequencing, data analysis and processing , issuance of test reports.
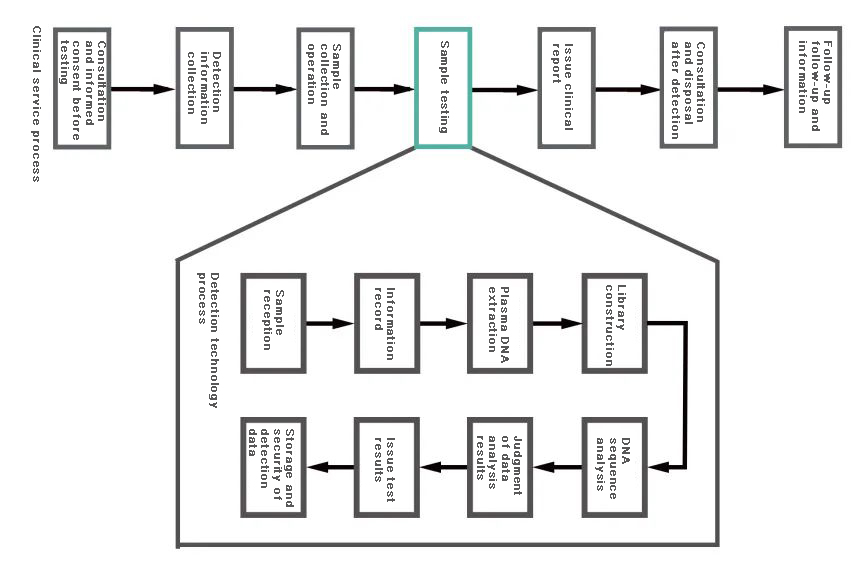
Figure 5 Clinical service process
For cfDNA extraction and library construction process, Allsheng can provide high-quality solutions.
cfDNA Automatic Extraction

Auto-Pure 24D Automatic Nucleic Acid Extraction System
■The maximum loading volume is 4ml, and the reaction system is 10ml, which can meet the needs of large-volume extraction
■The minimum elution volume is 50μl, effectively ensuring nucleic acid concentration
■Reagent tank single strip design, 1-24 samples can be freely selected
Nucleic Acid Concentration Quantification

Fluo-200/800 Fluorometer
■Can detect up to 8 samples per run
■Accurate quantification and high accuracy with only 1-20 μL samples
■The lowest detection limit can reach 0.5 pg/μL (dsDNA)
Automated Library Preparation

Auto-NGS 100R Automated NGS Library Preparation Workstation
■Max throughput: 24 samples
■8-channel pipetting (1- 200 μL)
■Built-in thermal cycler module to automate the amplification process
Reference
[1] Report on the Prevention and Treatment of Birth Defects in China (2012) [R]. Beijing: Ministry of Health of the People's Republic of China, 2012
[2] Liu Chunlin, Chen Peisong, He Xiaohong, et al. Application of Non-invasive DNA Screening in Peripheral Blood of 4723 Pregnant Women in Prenatal Diagnosis [J]. China Maternal and Child Health, 2020, 35(21): 4055-4059.]
[3] Wu L, Wu Y, Zou S, et al. Eliciting Women’s Preference for Prenatal Testing in China: a Discrete Choice Experiment [J]. BMC Pregnancy and Childbirth, 2020, 20(1): 1-8.
[4] O'Leary P, Maxwell S, Murch A, et al. Prenatal Screening for D Own Syndrome in A Ustralia: Costs and Benefits of Current and Novel Screening Strategies [J]. Australian and New Zealand Journal of Obstetrics and Gynaecology, 2013, 53(5): 425-433.
[5] Ashoor G, Syngelaki A, Poon L C Y, et al. Fetal Fraction in Maternal Plasma Cell‐free DNA at 11–13 Weeks' Gestation: Relation to Maternal and Fetal Characteristics [J]. Ultrasound in Obstetrics & Gynecology, 2013, 41(1): 26-32.
[6] Gil M M, Accurti V, Santacruz B, et al. Analysis of Cell‐free DNA in Maternal Blood in Screening for Aneuploidies: Updated Meta‐analysis [J]. Ultrasound in Obstetrics & Gynecology, 2017, 50(3): 302-314.

 Biological Sample Preparation
Biological Sample Preparation
 Life Science Detection Products
Life Science Detection Products
 POCT Detection & Reagent
POCT Detection & Reagent
 Automation & Liquid Handling
Automation & Liquid Handling
 Laboratory Instrument
Laboratory Instrument
 Reagent & Consumable
Reagent & Consumable
 Others
Others
 OEM/ODM
OEM/ODM












 Release time:2022-09-16
Release time:2022-09-16
 Source:
Source:
 Pageviews:3859
Pageviews:3859

















 + 86 571-88859758
+ 86 571-88859758 sales@allsheng.com
sales@allsheng.com



