How to Use Feyond-F100 Fluorescence Microplate Reader to Detect Recombinant Factor C Endotoxins
Bacterial endotoxins are a type of internal toxin produced by bacteria, typically present in lipopolysaccharides in the bacterial outer membrane. When bacteria die or the bacterial wall is damaged, these lipopolysaccharides are released into the surrounding environment and are very toxic. In the human body, the blood circulatory system transmits them to various organs and tissues, leading to excessive inflammatory reaction of the immune system and pathological changes such as cell apoptosis and necrosis. Endotoxins are widely present and difficult to inactivate. Sensitive and reliable endotoxin analysis techniques are very necessary in the pharmaceutical and medical device industries.
Tachypiens amebocyte lysate is widely used to detect bacterial endotoxins in pharmaceutical products, but the scarcity of horseshoe crab resources restricts the production of tachypiens amebocyte lysate. Recombinant Factor C (rFC), as a substitute for tachypiens amebocyte lysate, is obtained through gene recombination expression. After binding with endotoxins, the activated recombinant factor C can cleave substrates and obtain free fluorophores, and the release of fluorophores is proportional to the concentration of endotoxins. Therefore, the endotoxin content can be quantified according to the fluorescence intensity.
For the application of recombinant factor C endotoxin fluorescence detection, Allsheng Feyond-F100 fluorescence microplate reader customizes the software analysis method, realizes standard curve fitting, sample concentration quantitation and recovery rate calculation, so as to achieve the integration of the experimental operation and result output.
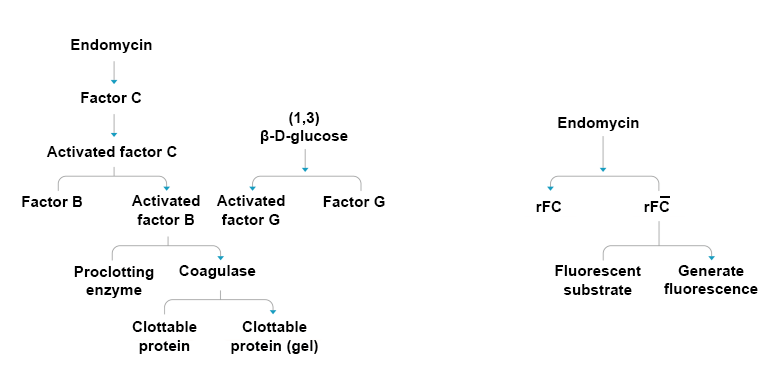
Figure 1 Comparison of the Endotoxin Detection Principles of Traditional Tachypiens Amebocyte Lysate (Left) and rFC (Right)
Experimental Material
Kit Name: Rhinogen® Recombinant Factor C Endotoxin Test Kit (Code: RAF-01/02)
Instrument: Allsheng Feyond-F100 fluorescence microplate reader
Experimental Method
After adding samples, the fluorescence detection values are respectively read after 0 h and 1 h incubation at 37 °C. The relative fluorescence unit (ΔRFU) of the negative control is used to calibrate the relative fluorescence unit (ΔRFU) of the standard to obtain the net fluorescence difference. The logarithm of net fluorescence difference is proportional to the logarithm of endotoxin concentration and linear within the range of 0.005 to 5.0 EU/mL.
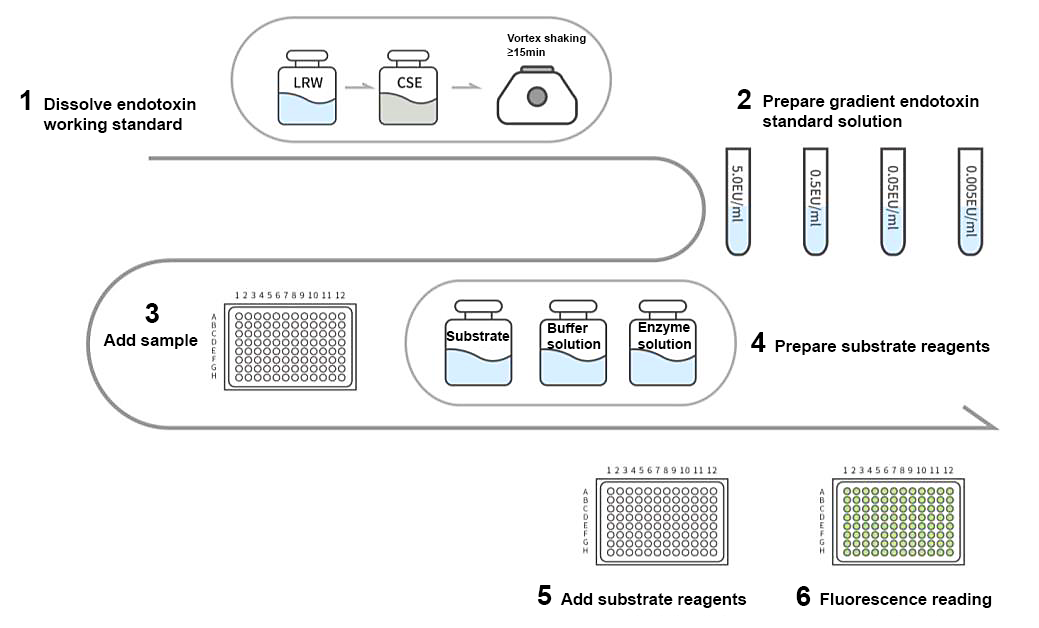
Figure 2 Operation Flowchart of Rhinogen® Recombinant Factor C Endotoxin Test Kit
Parameter Settings of Fluorescence Microplate Reader
Table 1 Detection Parameter of Microplate Reader
Detection mode | Fluorescence |
Detection type | Factor C |
Detection wavelength | EX/EM: 380/440 nm |
PMT gain | Automatic |
Integration time | 40 μs |
•Customize the Recombinant Factor C Software Interface
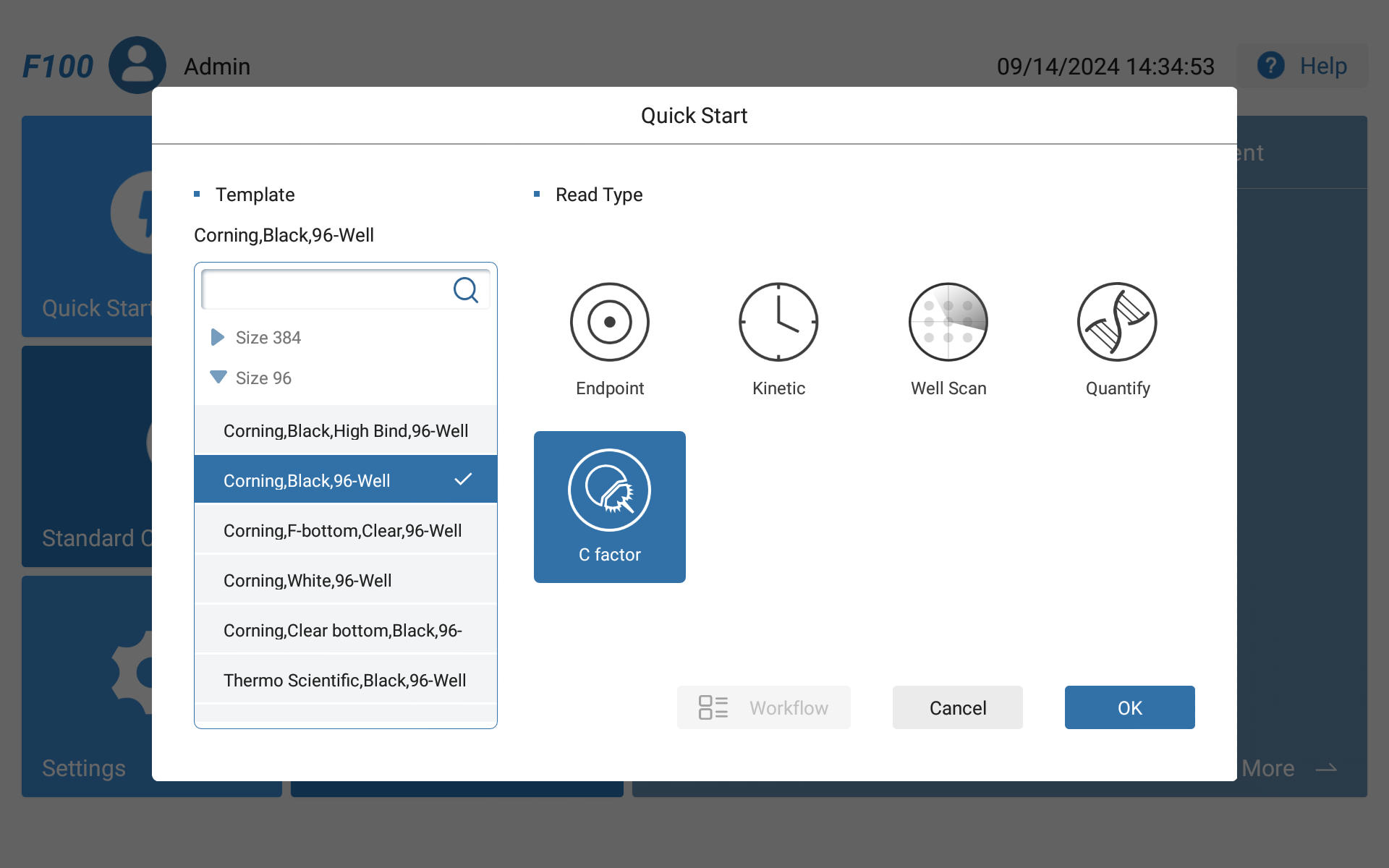
•Standard and Sample Layout and Parameter Interface
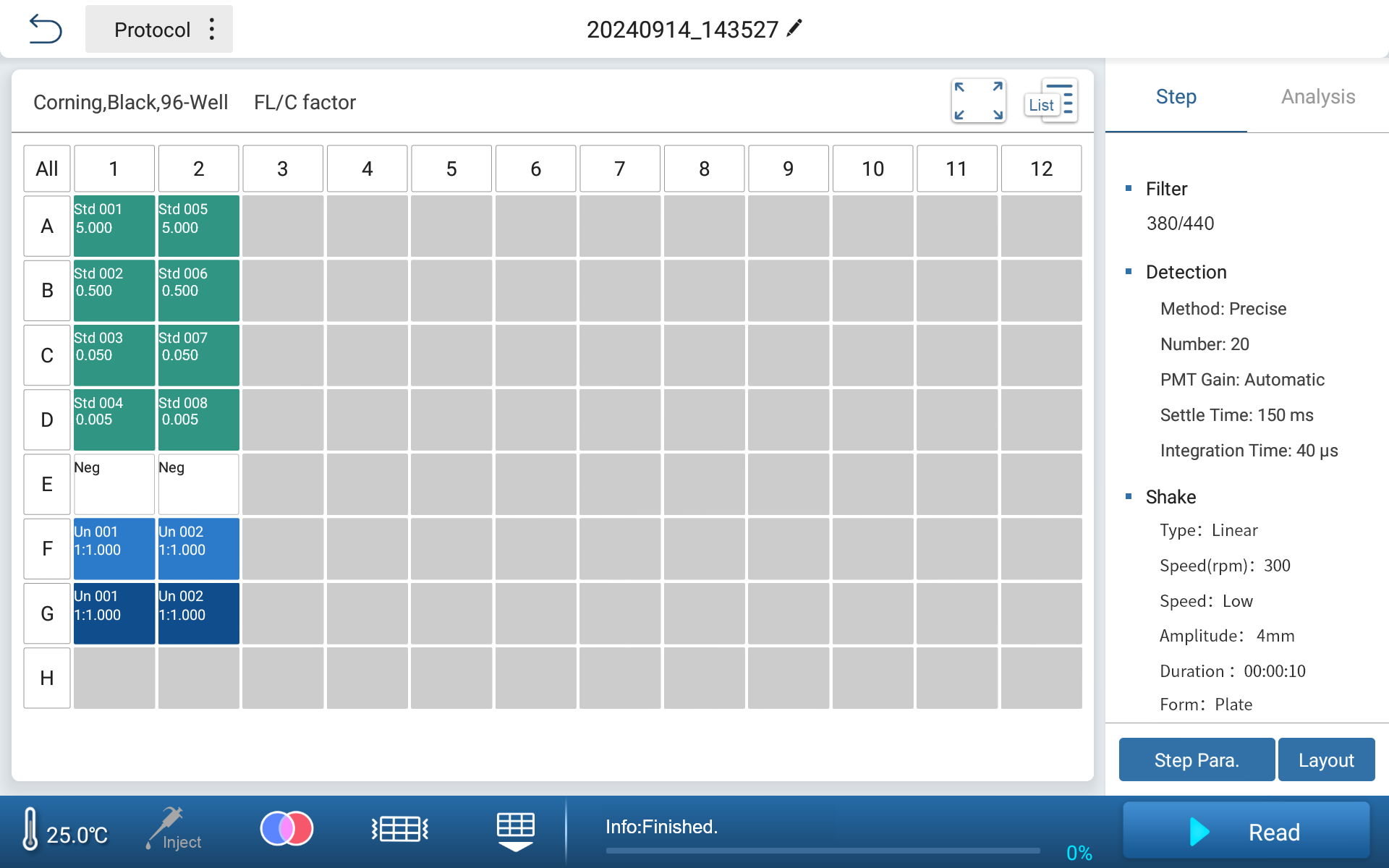
Experimental Result
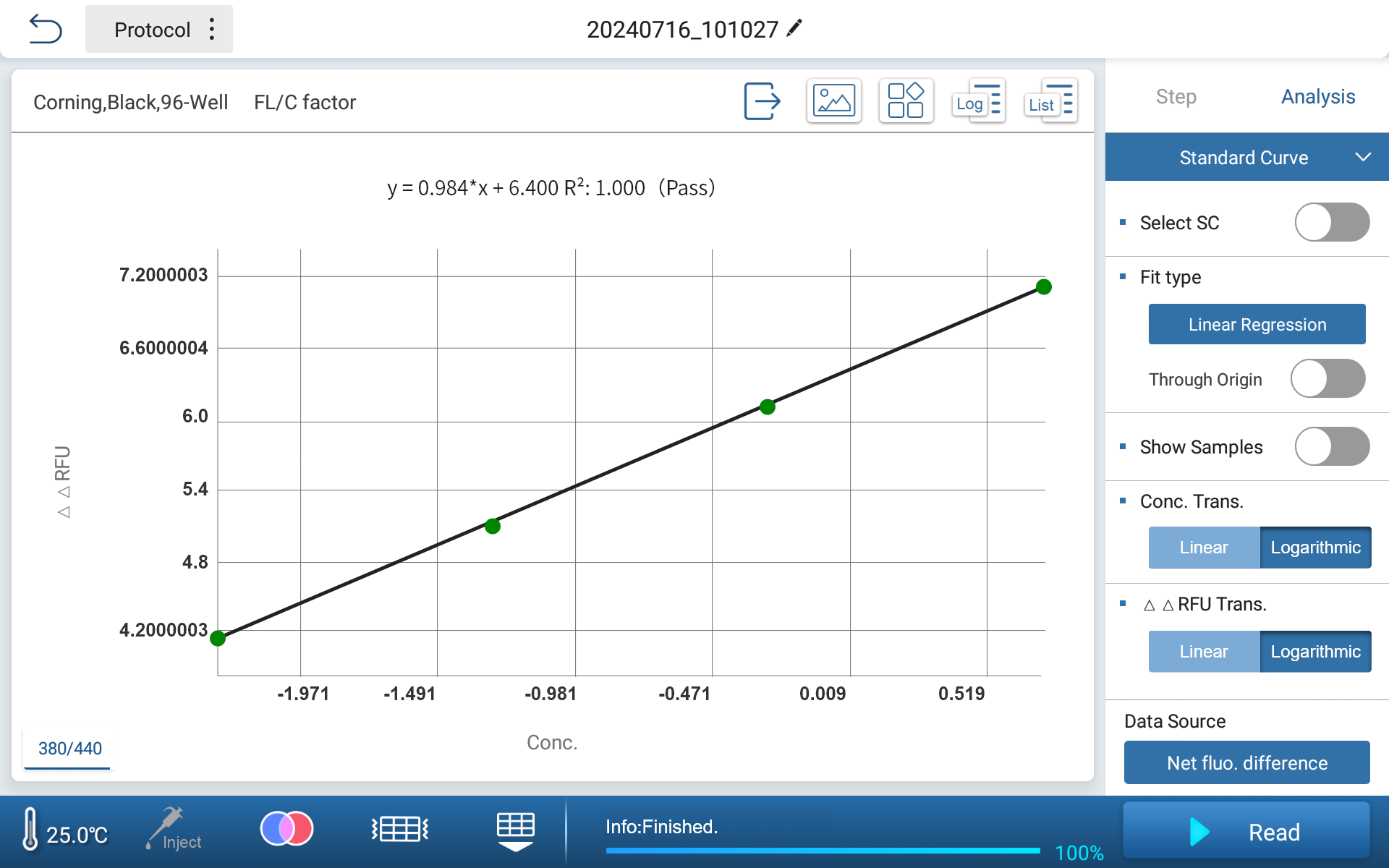
Feyond-F100 fluorescence microplate reader meets the test requirements of Rhinogen® recombinant factor C endotoxin test kit. The software can automatically fit standard curves for net fluorescence difference (log value) and concentration (log value). Four gradients of endotoxin dilutions (concentration from 0.005 EU/mL to 5 EU/mL) are used for testing, with an R2 value of 0.99977. The standard curve shows good linearity within this extended concentration range.
•Standard Curve and Sample Concentration Display Interface
•Sample Recovery Rate Determination Interface
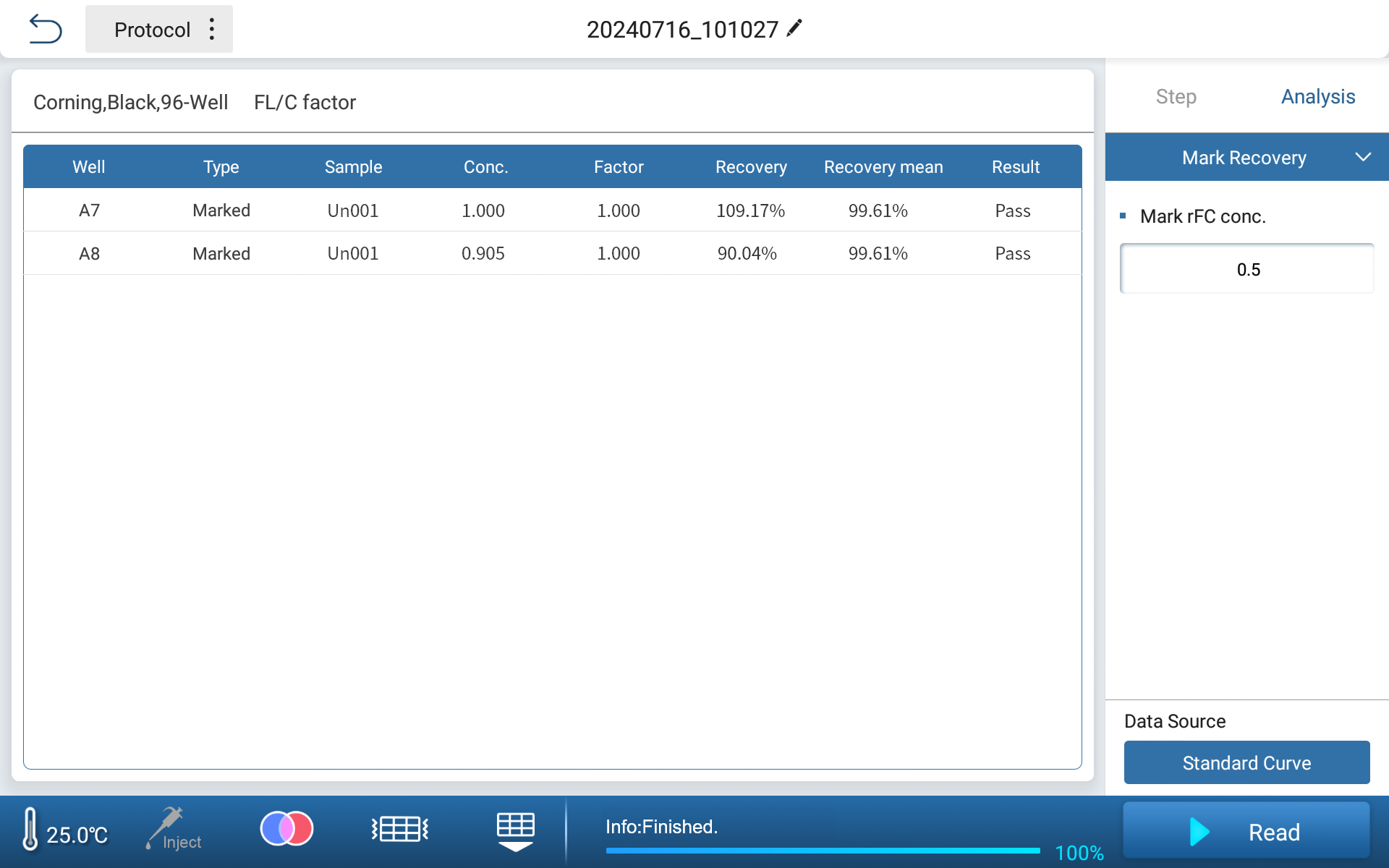
Feyond-F100 fluorescence microplate reader can be equipped with customized filters for recombinant factor C endotoxin detection experiments. The independent algorithm analysis software can meet the experimental requirements, which can conveniently and quickly realize the sample data analysis and provide intuitive result display.

 Biological Sample Preparation
Biological Sample Preparation
 Life Science Detection Products
Life Science Detection Products
 POCT Detection & Reagent
POCT Detection & Reagent
 Automation & Liquid Handling
Automation & Liquid Handling
 Laboratory Instrument
Laboratory Instrument
 Reagent & Consumable
Reagent & Consumable
 Others
Others
 OEM/ODM
OEM/ODM












 Release time:2024-09-20
Release time:2024-09-20
 Source:
Source:
 Pageviews:2057
Pageviews:2057















 + 86 571-88859758
+ 86 571-88859758 sales@allsheng.com
sales@allsheng.com



