1. The Origin of Carcinoembryonic Antigen
Carcinoembryonic antigen (CEA) is a glycoprotein with a molecular weight of 150,000 to 300,000 Daltons. In 1965, a tumor related antigen was first extracted by Canadian scholars from colon adenomas and fetal intestines. Mainly present in the rectum, colon cancer tissue, and embryonic intestinal mucosa. Because this antigen also exists in the gastrointestinal, liver, and pancreatic tissues of 2-6 month old embryos, it is named carcinoembryonic antigen, also known as embryonic antigen (EA) or fetal antigen (FA)[1].
2. Clinical Significance of CEA
CEA in human serum belongs to a broad-spectrum tumor marker, and its concentration is closely related to various organ cancers, especially colon cancer. CEA is present in the serum of patients with malignant tumors of endodermal origin, such as colon, rectum, esophagus, stomach, liver, and pancreas, and its content is significantly higher than that of non tumor patients[2]. The serum CEA level in tumor patients is related to the progression of the tumor, and tracking and measuring CEA is helpful for postoperative observation and judgment of surgical success or failure.
3. Detection Techniques for CEA
The methods used for detecting CEA include ELISA, RIA, CLIA, ECLIA and TRFIA. TRFIA is a widely recognized and most promising non radioactive immunolabeling technique[3] that developed rapidly in the 1980s. TRFIA has advantages such as high sensitivity, strong specificity, good stability, wide detection range, and non radioactivity.
ALLSHENG Feyond-A400 multi-mode microplate reader adds a new TRF detection function based on Feyond-A300, which can detect TRFIA experiments.
4. Material
Carcinoembryonic antigen assay kit (Guangzhou DARUI)
Feyond-A400 multi-mode microplate reader
5. Method
Standard Formula
Prepare CEA standards at concentrations of 1, 5, 10, 100, and 560 ng/mL.
Instrument Parameter Setting
| Reagent wavelength | EX/EM: 365/612 |
| PMT gain | Medium High |
| Settle time | 150 ms |
| Integration time | 400 μs |
| TRF delay | 100 μs |
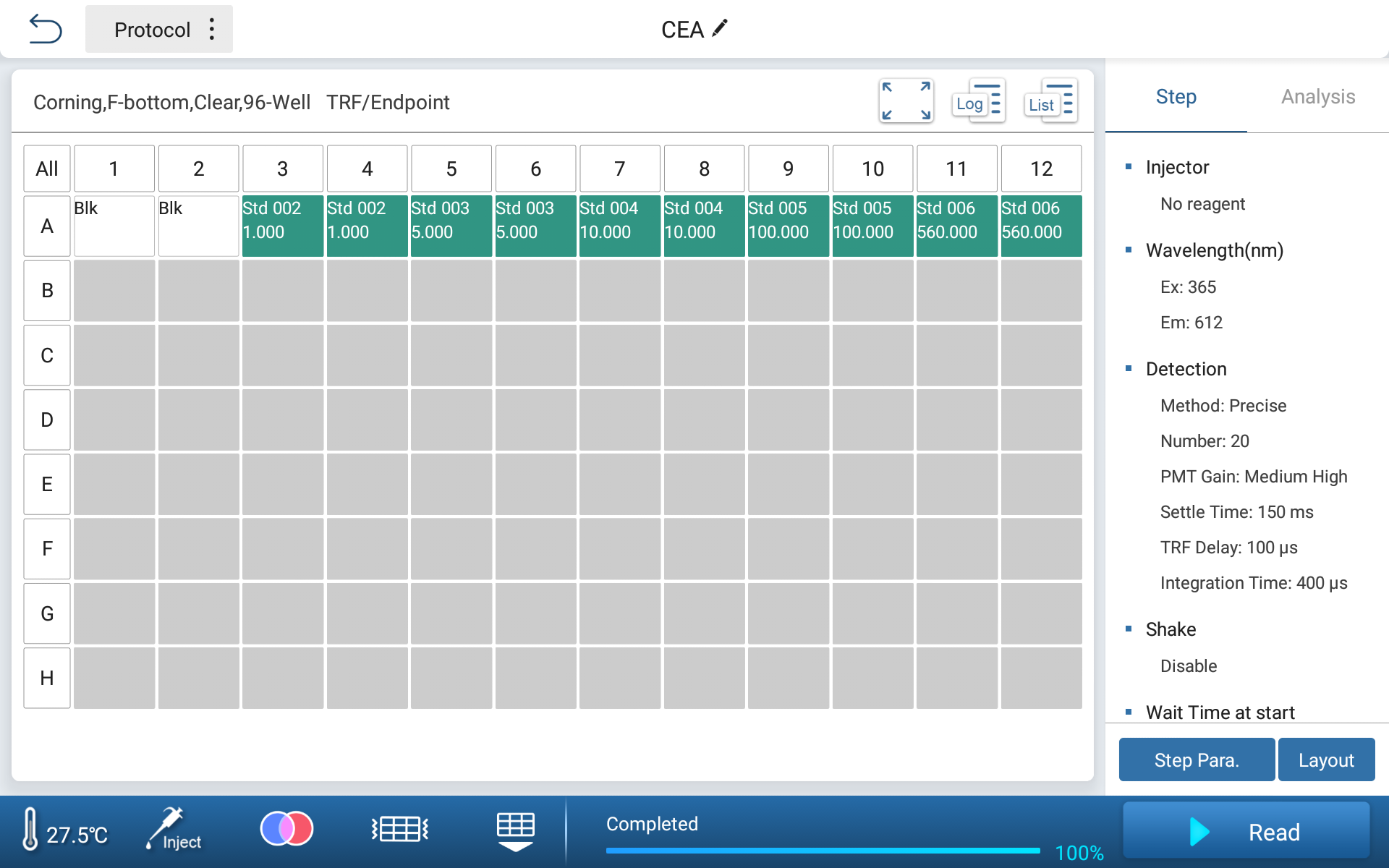
Figure 1. Basic Parameter Setting
6. Result
Analysis Parameter
| Curve fitting method | Spline function |
| X-axis | LOG |
| Y-axis | LOG-B |
The built-in data processing software of Feyond-A400 can directly fit the standard curve of CEA standard concentration and fluorescence intensity, and obtain the curve using cubic spline fitting method.
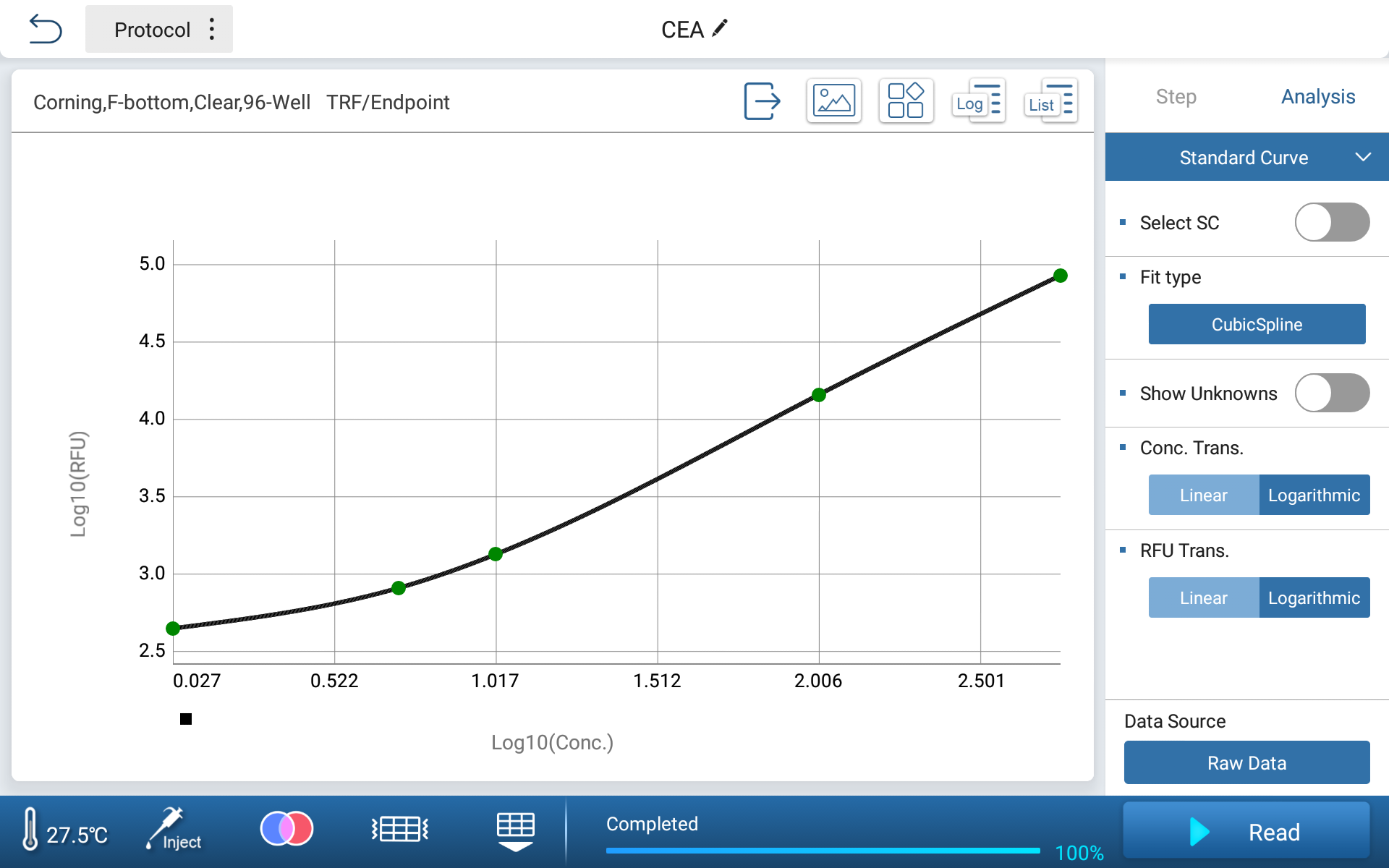
Figure 2. Instrument Generated CEA Standard Curve
Based on the obtained standard curve, the standard concentration can be inversely calculated, and the deviation from the theoretical value can be within 4%.
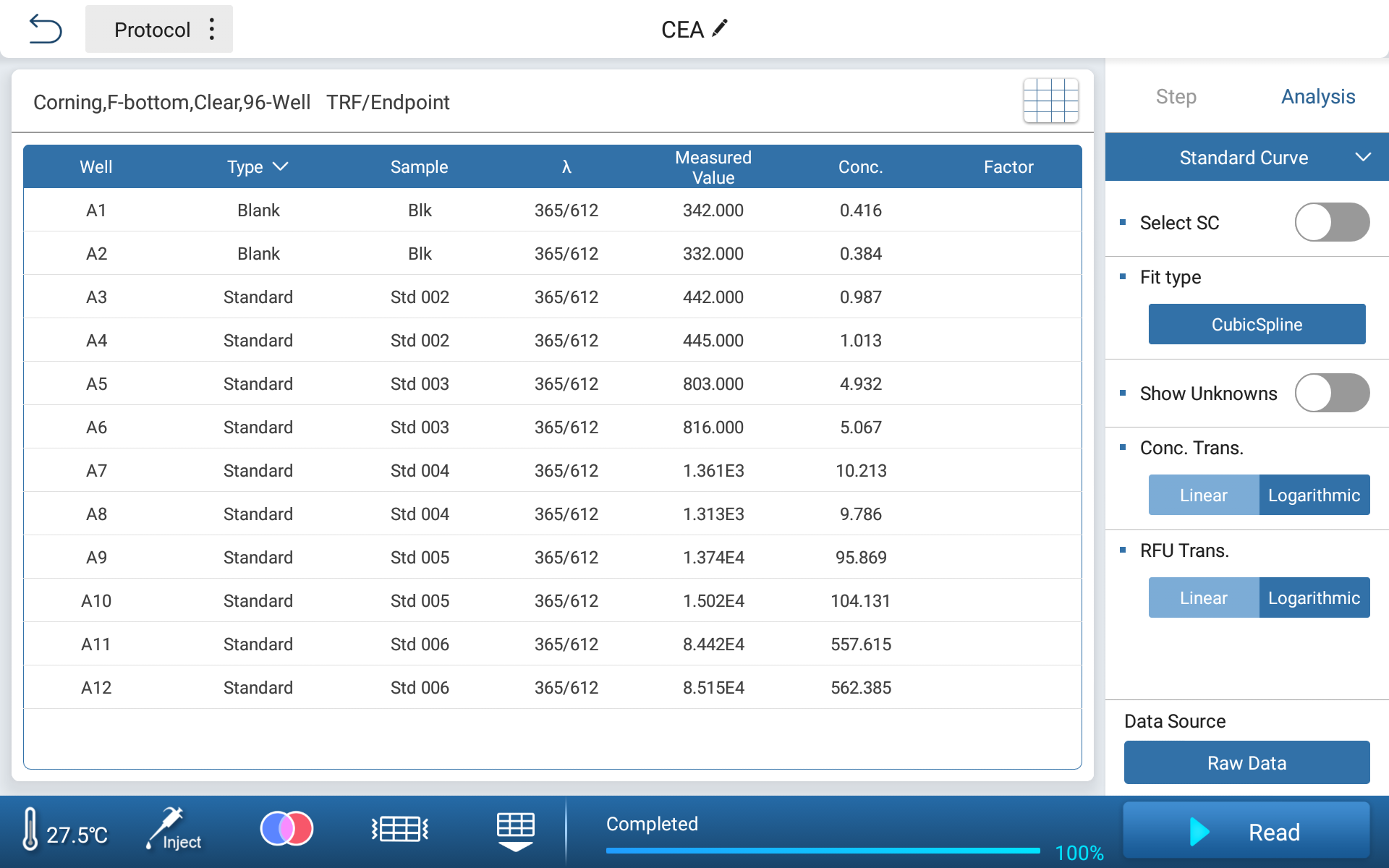
Figure 3. Inverse calculation of concentration for standard products
Compared with conventional fluorescence immunoassay methods, TRFIA can effectively avoid interference from self fluorescence, greatly improve the sensitivity and accuracy of detection, and has been widely used in fields such as medicine, food detection, and veterinary drug residue detection. The TRF detection function of Feyond-A400 can achieve the detection of TRFIA related applications.
Reference:
[1] Shi Yun, Wang Zhenjun, Wang Bin. Research Progress on the Relationship Between Carcinoembryonic Antigen and Clinical Diseases [J]. Chinese Journal of Physicians, 2005 (S1): 4. DOI: CNKI: SUN: DDYS.0.2005-S1-341
[2] Li Bilian, Liu Cui, Tong Yanli, et al. Progress in the Detection of Carcinoembryonic Antigen (CEA) [J]. Journal of Molecular Diagnosis and Treatment, 2008, 20 (002): 48-51
[3] Qiao Yanhong, Gu Zeliang, Zhang Jian, et al. Establishment and Application of Trfia Method for Hepatitis B Virus Surface Antibody [J]. Labeled Immunoassay and Clinical, 2007,14 (1): 3. DOI: 10.3969/j.issn.1006-1703.2007.01.009

 Biological Sample Preparation
Biological Sample Preparation
 Life Science Detection Products
Life Science Detection Products
 POCT Detection & Reagent
POCT Detection & Reagent
 Automation & Liquid Handling
Automation & Liquid Handling
 Laboratory Instrument
Laboratory Instrument
 Reagent & Consumable
Reagent & Consumable
 Others
Others
 OEM/ODM
OEM/ODM












 Release time:2023-09-21
Release time:2023-09-21
 Source:
Source:
 Pageviews:3150
Pageviews:3150












 + 86 571-88859758
+ 86 571-88859758 sales@allsheng.com
sales@allsheng.com



